Every fluorine atom will form a bond with the central atom, which means there will be four bonds in the molecule structure using up four valence electrons of fluorine atoms and 4 electrons of the sulfur atom. So now, eight valence electrons are used, reducing the number of valence electrons from 34 to 24. Explanation: Sulfur has six valence electrons. Valence electrons are the outermost electrons which, therefore, are located on the highest energy levels. Neutral sulfur has 16 electrons because its atomic number is 16. Also, how many valence electrons does the sulfur atom have? 6 valence electrons. This is the same amount of electrons as the number of valence electrons that oxygen atoms have on their own, and as such both of these oxygen atoms have a formal charge of zero. The two oxygens with the single bonds to sulfur have seven electrons around them in this structure (six from the three lone pairs and one from the bond to sulfur). Total valence electrons = 6 + 24 + 2 = 32; Total valence electrons pairs. Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells. Total electron pairs are determined by dividing the number total valence electrons by two. For, H 2 SO 4 molecule, Total pairs of electrons are 16. Center atom and sketch of H 2 SO 4.
- Number Of Valence Electrons In Sulfur Hexafluoride
- Valence Electrons Calculator
- Number Of Valence Electrons In Sulfur Dioxide
- Number Of Valence Electrons In Sulfur Atom
- Number Of Valence Electrons In Sulfur Trioxide
Thiosulfate ion contains two sulfur atoms and three oxygen atoms. In lewis structure of S2O32- ion, there is -2 charge and oxygen atoms should hold them. Total valence electrons of sulfur and oxygen atoms are used to draw the structure.

Thiosulfate ion | S2O32-
Thiosulfate ion is one of the oxyanion of sulfur. Two sulfur atoms exist at two different oxidation states as +4 and +6. Also, overall charge of thiosulfate ion is -2. Therefore, there should be charges in atoms in thiosulfate ion.
Number Of Valence Electrons In Sulfur Hexafluoride
Lewis structure of S2O32-
Steps of drawing lewis structure of S2O32-
Following steps are required to draw the S2O32- lewis structure and they are explained in detail in this tutorial.
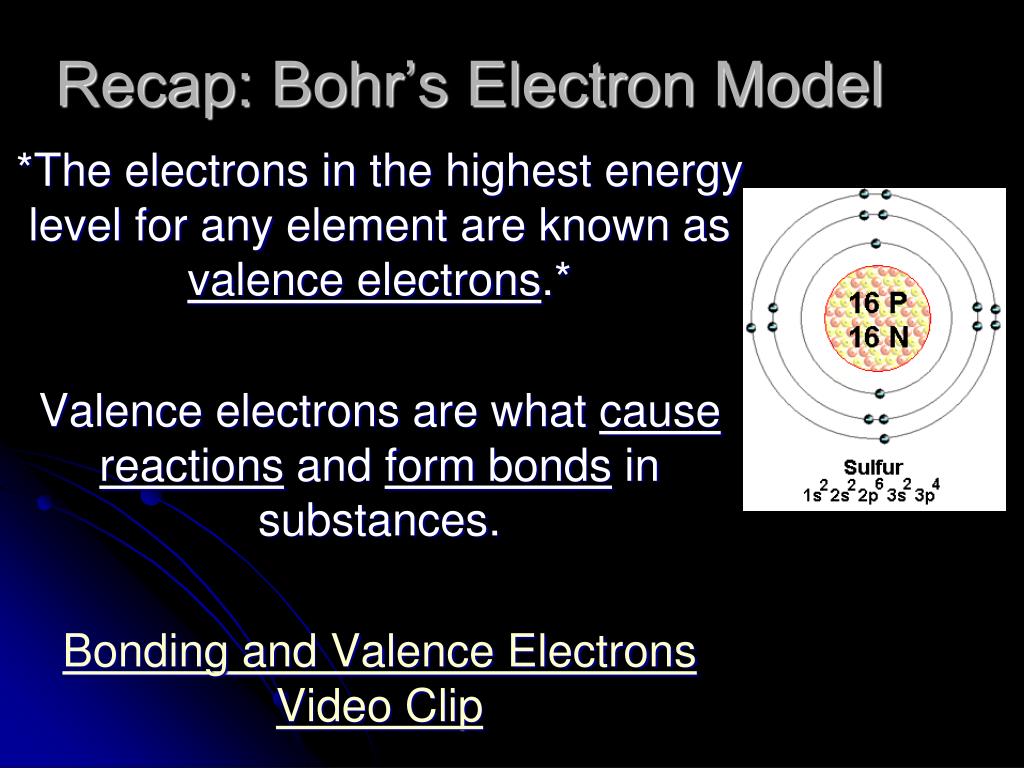
- Find total number of electrons of the valance shells of sulfur and oxygen atoms
- Total electrons pairs
- Center atom selection
- Put lone pairs on atoms
- Check the stability and minimize charges on atoms by converting lone pairs to bonds.
Drawing correct lewis structure is important to draw resonance structures correctly
Valence Electrons Calculator
Total number of electrons of the valance shells of S2O32-
Both Sulfur and oxygen atoms are located at VIA group in the periodic table. So, oxygen and sulfur atoms have six electrons in their valence shell.
- Total valence electrons given by two sulfur atoms = 6 * 2 = 12
There are three oxygen atoms in S2O32- ion, Therefore,
- Total valence electrons given by oxygen atoms = 6 *3 = 18
There are -2 charge on S2O32- ion. Therefore there are two more electrons which comes from outside to contribute to the total valence electrons.
- Total valence electrons = 6 + 24 + 2 = 32
Total valence electrons pairs
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells
Total electron pairs are determined by dividing the number total valence electrons by two. For, S2O32- ion, Total pairs of electrons are 16.
Center atom of S2O32- ion
To be the center atom, there are requirements.
Number Of Valence Electrons In Sulfur Dioxide
- ability of having greater valance is important.
- Being the least electronegative element
Therefore sulfur has the more chance to be the center atom (See the figure) because sulfur can show valance of 6 and electronegativity of sulfur is less than oxygen. Maximum valence of oxygen is two. So, now we can build a sketch of S2O32- ion.
Number Of Valence Electrons In Sulfur Atom
Around center sulfur atom, there is three oxygen atom and other sulfur atom.
Lone pairs on oxygen and sulfur atoms
- There are three S-O bonds and one S-S bond in the above sketch. Therefore only twelve (16-4 = 12) valence electrons pairs are remaining to complete the lewis structure.
- First, mark those thirteen valence electrons pairs as lone pairs on outside atoms (on oxygen atoms and sulfur atom). One oxygen atom and sulfur atom (located at outside) will take three lone pairs following the octal rule (oxygen atom cannot keep more than eight electrons in its valence shell).
- For three oxygen atoms and outside sulfur atom twelve electrons pairs are spent. Now all electron pairs (16) are spent. There is no electron pairs to mark on sulfur atom.
Charges on atoms
After, marking electron pairs on atoms, we should mark charges of each atom. Each oxygen atom will get a -1 charge and sulfur atom get a +2 charge. The overall charge of ion is ( -1*4 + (+2) ) = -2.
Check the stability and minimize charges on atoms by converting lone pairs to bonds
When charges exist everywhere (on atoms) in the ion or molecule, that structure is not stable. We should try to reduce charges on atoms as much as possible. Now, we are going to learn how these facts will affect on sulfate ion.
- Oxygen atoms should hold negative charges because electronegativity of oxygen is higher than sulfur. Otherwise, we can say, ability of holding negative charges is great in oxygen atoms than sulfur atoms.
- The drawn structure is not a stable one because all atoms have charges.
- Now, we should try to minimize charges by converting lone pair or pairs to bonds. So convert one lone pair of outside sulfur atom to make a new S-S bond.
- Now there is a double bond between two sulfur atoms. Now, there are three S-O single bonds between sulfur atom and other three oxygen atoms.
You see charges of atoms are reduced in new structure. Now, there is no charge in outside sulfur atom and charge of center sulfur atom is reduced from +2 to +1. Charges on oxygen atom are remaining as same. However, charges of atoms are reduced. So we have an stable ion than out previous one.
Can I reduce charges of atoms furthermore?
You should know, sulfur can keep more than eight electrons in its last shell. Therefore we can convert one more lone pair of an oxygen atom to a bond to make a double bond between center sulfur atom and oxygen atom.
In new structure, charges of atoms are reduced than previous structure. Now there are no any charge on both sulfur atoms and one oxygen atom. Also, only two oxygen atoms have -1 negative charges. Now you understand this structure of S2O32- is more stable than previous two structures. So, this structure has more chance to be the lewis structure of S2O32- ion.
Lewis structure of S2O32- (thiosulfate) ion
Questions

Is number of lone pairs similar in S2O32-- and SO42- lewis structures?
Yes. Compare both lewis structures. Otherwise, we can think an oxygen atom of sulfate ion is replaced by a sulfur atom. Because both sulfur and oxygen belongs to group 6, their valence electrons in last shell is similar.
Can I draw the lewis structure of S2O32- putting a negative charge on sulfur atom?
Usually we dont put negative charges on electropositive atoms when there are strong electronegative atoms like oxygen. Oxygen's electronegativity is higher than sulfur. So we should put our negative charges on oxygen. Those structures are most stable ones.
what is the charge on oxygen atom in lewis structure of thiosulfate ion?
Number Of Valence Electrons In Sulfur Trioxide
Two oxygen atoms have negative charges. Each od them have -1 charge and overall charge of thiosulfate ion is-2. There is no charge on remaining oxygen atom.
structure of s2o32-
Lot of students struggle to draw lewis structure of S2O32- because there is two sulfur atoms. They cannot think, how sulfur atoms are joint with oxygen atoms in this molecule. There is no doubt to select sulfur as the center atom. So rest of oxygen atoms and sulfur atom should be around the center sulfur atom.
Related Tutorials
SO32- lewis structure and resonance structures
NO3- lewis structure
NO3- resonance structures
NO2- lewis structure
N2O lewis structure, resonance structures
N2O5 resonance structures
Resonance structures examples
Nitrogen dioxide acidity




